A new study from Ghent, Belgium, discusses the difference between pain sensation and pain thresholds between subjects with genetically-confirmed classical Ehlers-Danlos syndrome (cEDS) and those without any form of Ehlers-Danlos Syndrome (EDS). The article, published in The Journal of Pain, discussing this research is “Sensory Profiling in Classical Ehlers-Danlos Syndrome: A Case-Control Study Revealing Pain Characteristics, Somatosensory Changes, and Impaired Pain Modulation” by Colman M, Syx D, De Wandele I, et al. This article will introduce the main findings of the study.
In general, pain is a misunderstood and under-researched phenomenon. In EDS, pain is one of the most commonly reported symptoms and existing treatments are, at best, partially effective. This study’s purpose was to find out the difference between the ways people with cEDS perceive pain as to understand how to potentially treat it.
Classical Ehlers-Danlos is one of the most common genetically-explained types of the Ehlers-Danlos syndrome. It affects an estimated 1 in 20,000 people while hypermobile Ehlers-Danlos syndrome (hEDS)–unable to be genetically explained yet–affects an estimated 1 in 5,000 people. Greater than 70% of individuals with cEDS self-reported pain as a symptom in other studies. No studies have been done specifically on pain sensations in cEDS or any other genetically confirmed type of EDS. A few studies have researched pain sensations in people with hEDS and hypermobility spectrum disorder (HSD), but inconsistent results have been reported. These differences could possibly be due to variations in the study’s parameters and testing methods.
Researchers are learning more about how the extracellular matrix (ECM) is involved in pain sensations. In people with heritable connective tissue disorders, like EDS, the ECM, specifically, the fibrillin that makes up part of the ECM, is compromised. It has been shown in mouse studies that the defects in the ECM could be one of the reasons that people with EDS have so much pain. Studies have also shown that pain-behavior and abnormal nerve distribution in the skin is present in mice with cEDS. This brought about this current study to find out if the somatosensory–that is, senses without a specific input organ, like heat and pressure, rather than something like the sense of sight or smell–alterations and pain sensations are altered in individuals with cEDS.
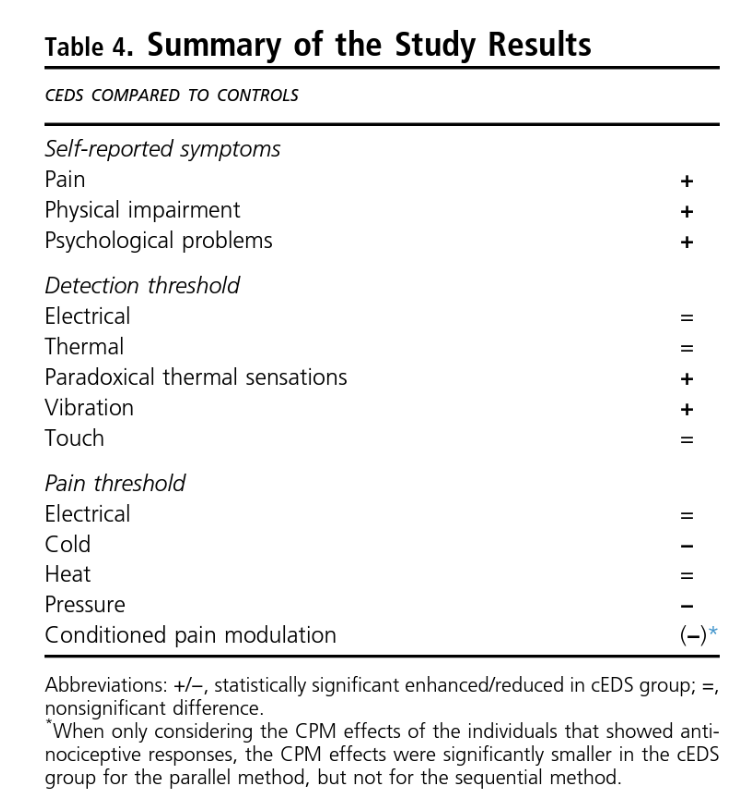
“Chronic pain is highly prevalent in cEDS, and the disease has a profound influence on the individual’s quality of life and emotional well-being,” say the study’s authors. “Somatosensory profiling in cEDS showed the presence of hyperalgesia [increased sensitivity to pain] for pressure and cold stimuli, hypoesthesia [decreased sensitivity to pain] for vibration stimuli, and alterations in thermal sensibility illustrated by the higher number of paradoxical thermal responses [unable to tell the difference between warmer and cooler] and impaired endogenous pain modulation [the way the central nervous system acts to increase or decrease pain].”
Study Details
To assess these potential alterations and changes, researchers used static and dynamic quantitative sensory testing (QST), which is “a systematic psychophysical test method used to measure sensory thresholds for pain, touch, vibration and temperature” (Physiopedia). To test participants’ sensitivity to various stimuli, investigators used different painful and non-painful stimuli, like a small electric shock or a piece of metal held to the skin that can increase or decrease in temperature. The researchers also used validated questionnaires to assess emotional and cognitive factors so as to provide as much data as possible about the study participants and their experiences with pain.
Nineteen individuals with genetically-confirmed classical Ehlers-Danlos syndrome (6 male, 13 female, ages 18-65) were recruited for this study. A control group matching in sex and age were recruited as well. This control group was screened for the following disqualifying criteria: generalized joint hypermobility (a Beighton score of 4/9 or higher); current chronic pain or a history of chronic pain; use of analgesics, antidepressants, anxiolytics, or antihypertensive medications; cardiac, respiratory, neurological or psychological issues; and pregnancy, breastfeeding or surgery in the last year.
One week before the experiment, participants filled out a series of questionnaires that captured socio-demographics, medical history, self-reported symptoms related to central sensitization, the presence of any depression or anxiety, self-reported levels of physical activity, as well as questionnaires to assess health status and disability. They were asked to avoid intense physical activity for 24 hours before the experiment. Previous studies have shown that exercise can reduce pain perception in health populations while it can induce or increase pain and other symptoms in chronic pain populations.
Participants were asked to refrain from caffeine, alcohol, and nicotine for three hours before the experimental procedures; a light meal was allowed. Participants with cEDS were asked to refrain from opioids for 24 hours, if medically allowed, and refrain from taking any short-acting pain medications or blood pressure medications until after the experiment.
On the day of the experiment, all participants completed a questionnaire to ensure 15 to 30 minutes of physical rest before the experiments as well as to assess current pain intensity and pain intensity over the past four weeks; pinpoint pain locations; presences of pain hypervigilance; and fear of movement. cEDS participants also did a questionnaire to assess the presence of neuropathic pain. Following, a clinical exam noted the following data: blood pressure, weight, length, and clinical signs of connective tissue fragility– generalized joint hypermobility, skin hyper-extensibility, presence of atrophic scars, and bruising.
Results of the questionnaire
Most individuals in the cEDS group experienced clinically relevant pain. In the cEDS group, the median score for current pain was 3/10 (on a Visual Analogue Scale [VAS]). Twenty-one percent of that group reported a pain score of greater or equal to five. When looking at the maximum pain over the last four weeks, the median score was 7/10 with 68% reporting a score greater or equal to five. When looking at the average pain intensity over the last four weeks, the median score was again 3/10 with 32% reporting greater or equal to five. The locations of the pain varied, but it was usually confined to a limited number of joints or the lower back.
The study’s authors wrote: “The majority [of the cEDS group] experienced some chronic pain/discomfort, mostly at a limited number of joints or the spine, but more generalized pain, as well as the absence of any pain, was also reported. The cEDS patients also experienced worse health-related quality of life with more limitations and impairment due to their disease.”
The cEDS group, as a whole, scored well above the cut-off (a score greater than 37) for fear of movement and had significantly higher individual scores on the fear and depression sub-scales than the individual scores of the non-cEDS group. However, when the individual scores were averages, the cEDS group’s score was below the cut-off for mild-to-moderate anxiety and depression. “The burden of disease also impacted the psychological well-being of the cEDS group with mild/moderate symptoms of anxiety and depression and more pain hyper-vigilance and kinesiophobia [fear of movement] compared to healthy controls…This contrasts with hEDS/HSD, in which psychiatric conditions, including depression and anxiety disorders, are more commonly observed,” stated the authors.
Static Quantitative Sensory Testing Results
Electrical detection thresholds (EDT) and electrical pain thresholds (EPT)
These thresholds were tested on the sural nerve, which is in the calf, behind the fibula. No differences were found for the electrical detection threshold (EDT) or electrical pain threshold (EPT) between the participants with cEDS and those without.
Thermal detection threshold (TDT) and thermal pain threshold (TPT)
These thresholds, which include the warm detection threshold (WDT) or the cold detection threshold (CDT), were tested on the upper one-third of the calf and on the muscle in the forearm that runs from the elbow to the wrist (brachioradialis muscle). There were no differences in the CDT and WDT, either on the leg or the arm, between the control group and the cEDS group.
Other factors of the TDT and TPT are the heat pain threshold (HPT) and cold pain threshold (CPT), tested in the same places as the WDT and CDT. The HPT did not differ between the two groups; however, the CPT was significantly higher in the cEDS group on the leg, but not on the arm, meaning the non-cEDS group could tolerate colder temperatures that the cEDS group.
Lastly, the participants with cEDS had a more difficult time telling the difference between hot and cold temperature changes (called the paradoxical thermal sensation or PTS). In both groups, it was more difficult to distinguish hot from cold on the leg, and the participants were more likely (60%) to interpret cold as heat.
Vibrational detection threshold (VDT)
A vibration device was applied to the participant’s inner ankle (the bone part that sticks out, called the medial malleoli) and the bony protuberance on the outside of the wrist (called the ulnar styloid process). This is where the vibrations detection threshold (VDT) was tested. Participants with cEDS had a significantly higher VDT at the ankle and a mildly increased VDT in the wrist.
Mechanical detection thresholds (MDT) and mechanical pain thresholds (MPT)
The mechanical detection threshold (MDT) was tested by applying pressure to the bottom of the big toe and the small, fleshy mound at the bottom of the pinky finger. No differences were found in the MDT between the cEDS group and the control group.
For mechanical pain threshold (MPT), pressure was placed on the upper 1/3 of the calf and on the muscle in the forearm that runs from the elbow to the wrist (brachioradialis muscle). The results of the MPT thresholds are reported with the dynamic Quantitative Sensory Testing (see next section).
The average of three measurements of each area were taken and then the average between the two areas was used as the final threshold value. For example, if a participant reported a 28.1/82.6, 26.7/80.1, and 24.2/75.6 degree C/F heat threshold in the calf, the average of those temperatures is 26.3 degrees C/79.4 degrees F. Then, the same participant reported 22.8/73.0, 23.7/74.7, and 26.2/79.2 degrees C/F on the arm. The average of those is 24.2 degrees C/75.6 degrees F. The researchers average 26.3 degrees C/79.4 degrees F and 24.2 degrees C/75.6 degrees F to get the final warm temperature threshold of 25.3 degrees C/ 77.5 degrees F. (This data is not real data from the study.)
Dynamic Quantitative Sensory Testing
Conditioned pain modulation (CPM)
A conditioned pain modulation (CPM) consists of “the evaluation of a painful test stimulus followed by a second evaluation either at the same time as a distant, painful conditioning stimulus (parallel method) or in series after the painful conditioning stimulus has been removed,” according to the NIH. It’s a test to find out if “pain inhibits pain”–that is to say, how does the endogenous nervous system regulate pain based on different stimuli? For example, study participants may be asked to drink an especially cold beverage (this is the “distant pain conditioning stimulus” or just the “condition”) while an electroshock is applied to their other hand (this is the “painful test stimulus” or just “stimulus). The researchers may ask the participant to rate the coldness of the beverage at the beginning of the test–this establishes a baseline score. Then, when the electroshock is applied, tell them how painful it is while drinking the cold beverage (parallel method, since the condition and stimulus are happening at the same time). The researchers may then ask the participant to finish the beverage, then apply a small shock, and ask the participant to rate the pain (sequential method, since the condition happened first, then the stimulus). The researcher will be able to see if drinking an extremely cold beverage affects how a person feels a different kind of pain or discomfort.
In the cEDS study, the participant’s non-dominant hand was immersed in heated water while the mechanical pain threshold was determined (as described above).
On average, there were no significant differences between groups when looking at the pain scores given after two minutes and six minutes of the hand being immersed in the hot water. During the sequential and parallel methods, the majority of participants (with cEDS and without) showed antinociceptive–that is, pain reducing or blocking–effects (between 61% and 94%, depending on group, body part, and method). There were not significant differences between the two groups in either the parallel or sequential tests. However, in participants who showed pain-relieving responses, the CPM effects were significantly smaller in both test locations in the parallel method, but not in the sequential method for the cEDS group.
Conclusion
The results indicate differences in the somatosensory alterations and differences in the actions of the endogenous nervous system (the system in the body that regulates pain) are indeed present in individuals with cEDS compared to those without cEDS. This study showed that people with cEDS don’t feel vibration stimuli until a higher threshold than those without cEDS. They also don’t feel temperature differences as precisely as those without cEDS, mistaking increases in temperature with decreases in temperature and vice versa. Their tolerance for cold and pressure were both lower as well. In the parallel CPM experiment, participants with cEDS “showed smaller antinociceptive response”–meaning, the presence of other pain didn’t diminish the sensation of the stimuli pain as much as it did in the non-cEDS group. This suggests some impairment of endogenous pain modulation.
Though these results may provide researchers with information on which to base future research, there are some limitations to this study. A small number of subjects (19 in each group) limits the extrapolation of the results to a larger population, and the self-reported surveys and reports of pain are subjective. No studies have been done regarding how reliable or valid self-reported questionnaires are in groups with chronic (musculoskeletal) pain or cEDS as there has been in the general population. There are also no previous studies to compare these results to. Existence of previous results could strengthen the findings of this study or call them into question. (The few hEDS/HSD studies in this area are mentioned previously in this article.) It’s clear that more studies need to be done to conclusively provide answers. The authors specifically suggest assessing small fiber arrangement in individuals with cEDS and more studies on proprioception to better understand and support these results.
“This study is the first to investigate pain in a systematic way in a genetically defined group of individuals with HCTD. Our findings indicate an altered neural function in cEDS, providing interesting new insights on the possible role of the ECM in the development and persistence of pain,” said the study’s authors. In time, the hope is that the way the body experiences pain–with or without the disruptions of cEDS–is better understood in order to provide treatment, relief, and possibly a cure for the millions of people who are affected by it daily.
Kate Schultz
January 2024


Please share the information about the organizations publishing the most current research on EDS. Which website is the most up-to-date date on EDS?
https://www.chronicpainpartners.com/interview-with-dr-norris-on-finding-the-genetic-causes-for-hypermobile-eds-and-much-more/